Funding Cuts in Medical Research: Impact on Patient Safety

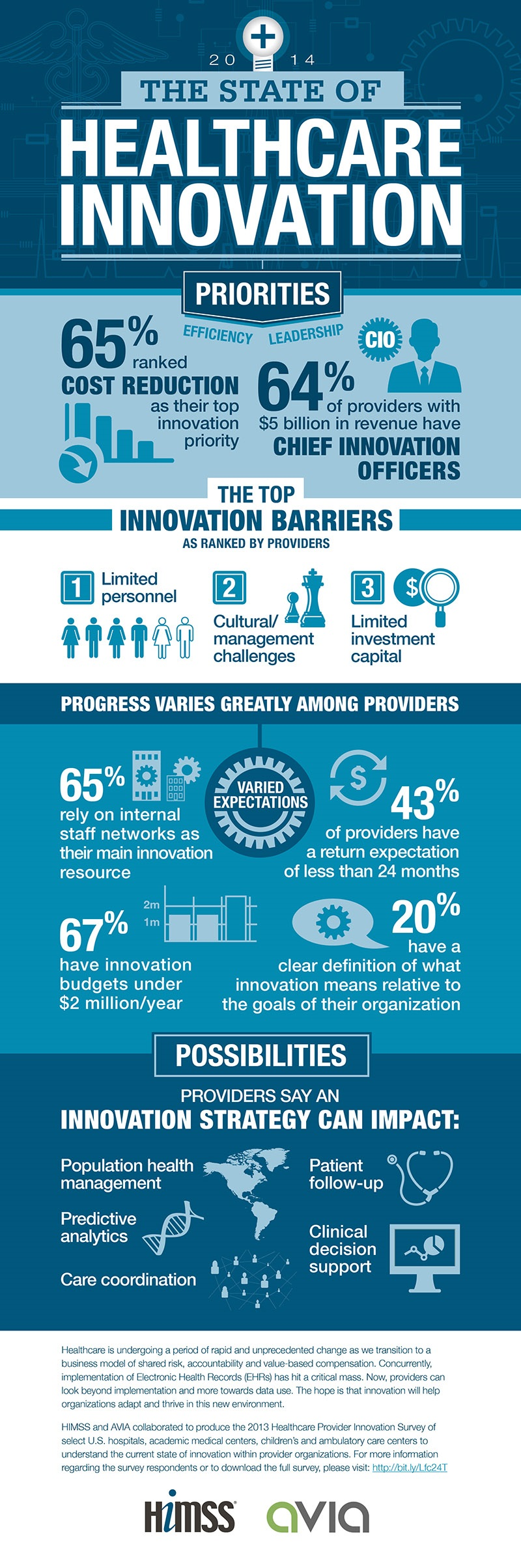
Funding cuts in medical research have become a pressing issue that threatens the vital safety protocols designed to protect patients involved in clinical studies. Recently, the Trump administration’s freeze of over $2 billion in federal research grants has reverberated throughout prestigious institutions like Harvard, disrupting essential oversight mechanisms. This halt in funding severely impacts not only the projects aimed at advancing healthcare but also jeopardizes the ethical standards upheld by research oversight bodies like Institutional Review Boards (IRBs). The implications of funding cuts extend far beyond just financial limitations; they can undermine patient safety and erode public trust in medical research. As researchers and institutions grapple with the fallout, the future of healthcare innovation and patient care hangs in the balance, emphasizing the critical importance of sustained medical research funding to uphold healthcare research ethics.
Recent reductions in financial allocations for medical studies pose significant challenges to maintaining the ethical standards needed to protect participants in clinical trials. Such budgetary constraints have a cascading effect, straining the resources that organizations like Institutional Review Boards (IRBs) rely on to ensure patient safety. A lack of adequate funding can hinder the rigorous oversight required for safe medical experimentation, potentially leading to a decline in the quality of healthcare research. It also raises concerns about the long-term viability of research ethics, as institutions struggle to uphold the necessary operational frameworks. Therefore, the ramifications of decreased financial support for scientific investigations raise alarming questions about the integrity and future of public health initiatives.
The Critical Role of Funding in Medical Research
Funding serves as the backbone of medical research, enabling institutions to explore innovative treatment options and ensure patient safety. In the landscape of clinical research, organizations rely heavily on federal funding, such as grants from the NIH, to support their regulatory and oversight activities. When federal funding is disrupted, as seen with the recent cuts, the entire infrastructure supporting medical research begins to crumble. The resulting financial strain can lead to severe delays and a reduction in the quality of oversight, ultimately jeopardizing participant safety across multiple sites.
The impact of funding cuts reaches far beyond immediate financial concerns. The suspension of grants can halt ongoing studies, disrupt research timelines, and prevent new trials from being initiated. This situation not only endangers the future of potential medical breakthroughs but also undermines public trust in clinical research. As research teams struggle to adapt to funding limitations, patient advocates worry that vulnerable populations may miss out on advancements in healthcare driven by rigorous scientific inquiry.
Impact of Funding Cuts on Patient Safety
Funding cuts in medical research pose a direct threat to patient safety and well-being. With the crucial role that IRBs play in ensuring ethical standards and protective measures for study participants, dwindling financial resources can severely limit their ability to function effectively. Without adequate funding, IRBs may face challenges in maintaining staff training, conducting thorough reviews, and managing the complexities of multi-site research. This threatens not only the individual participants’ rights but also the integrity of the studies themselves.
Moreover, the long-term implications of these funding cuts can exacerbate existing healthcare disparities. When research funding is contingent on federal support, underserved communities may be particularly affected, as there will be fewer resources allocated for studies focused on their unique health challenges. Clinical trials are vital for understanding diseases that disproportionately affect certain populations, and the lack of funding for equitable research can lead to inadequate treatment options and a persistent cycle of neglect for those communities.
Institutional Review Boards and Their Importance
Institutional Review Boards (IRBs) are an essential component of the medical research landscape, providing crucial oversight to protect the rights and welfare of participants. IRBs analyze study protocols to ensure ethical standards are upheld, evaluating informed consent processes and the potential risks to subjects. The operational effectiveness of IRBs hinges on funding, as financial support allows them to recruit knowledgeable staff and implement comprehensive training programs. Without sufficient funding, IRBs may struggle to fulfill their mission, compromising the quality of research oversight.
As we witness a trend of funding cuts, the implications for patient safety become increasingly serious. Fewer resources mean that IRBs may not be able to conduct thorough reviews or respond promptly to adverse events, leading to gaps in patient protection. This scenario is particularly concerning in multidisciplinary research, where oversight coordination is critical. The integrity of clinical trials and the well-being of participants depend on robust IRB processes that can only be maintained with stable funding.
Federal Regulations and Research Funding
Federal regulation plays a significant role in ensuring the ethical conduct of medical research, primarily through the establishment of funding mechanisms that support IRB functions. Research entities often rely on federal grants to cover the costs associated with compliance, ensuring that research meets the ethical standards required for human participants. The recent funding cuts have raised significant concerns regarding the capacity of IRBs to uphold these regulations, indicating a need for renewed focus on the importance of sustained financial support for ethical research.
The need to adapt federal regulations to current challenges in research funding can no longer be ignored. As the landscape of healthcare and research continues to evolve, so too must our approaches to ensure the safety of participants. The integration of funding stability within oversight mechanisms is critical; without it, the repercussions could endanger not only individual participants but also our collective advancements in medical knowledge.
Long-term Consequences of Stopping Medical Research Funding
Halting medical research funding presents long-term consequences that ripple beyond immediate financial implications. As research projects grind to a halt, the mission to address pressing health issues takes a back seat, especially for conditions that currently lack effective treatments. The resulting gaps in research can lead to a stagnation of knowledge and innovation, with lasting effects on societal health outcomes.
Furthermore, when funding is inconsistently allocated, the very framework that underpins healthcare research ethics and patient safety is compromised. As researchers and IRBs face increasing pressure to adapt to financial limitations, the potential for ethical oversights grows. The inability to maintain rigorous standards can undermine public confidence and trust in healthcare research as a whole, leading to broader concerns about the integrity and purpose of clinical studies.
Collaborative Research and its Funding Challenges
Collaboration across institutions is essential for advancing medical research, yet funding challenges pose significant barriers to these partnerships. The recent cuts to federal funding, particularly affecting programs like SMART IRB, have made it more difficult for multiple institutions to come together on clinical research initiatives. Collaboration requires robust support and funding to streamline processes and ensure compliance with ethical standards, something that is becoming increasingly elusive as budgets shrink.
The ability to establish a collaborative research environment is vital for fostering innovation. Yet, these funding restrictions can lead to fragmentation in research efforts, stifling the shared learning and development opportunities that emerge from diverse teams working together. Ultimately, the future of collaborative medical research hangs in the balance, highlighting an urgent need for advocates to push for renewed investment in funding mechanisms that support joint initiatives and patient safety.
Enhancing Ethics in Healthcare Research
As funding for healthcare research diminishes, the ethics surrounding medical studies become even more critical. The protection of research participants relies heavily on established ethical frameworks, which require adequate resources to implement effectively. A lack of funding can strain these ethical infrastructures, leaving room for potential exploitation and regulatory breaches. Upholding the principles of healthcare research ethics is paramount, and without sufficient funding, we risk compromising the very integrity of the research process.
Moreover, a commitment to enhancing ethics in healthcare research is essential not only for protecting current participants but also for rebuilding trust with the broader public. Providing transparency and demonstrating a steadfast commitment to ethical practices requires investment in ethical training and oversight mechanisms that may falter under financial distress. Funders and policymakers must recognize the importance of sustaining not only financial support but also fostering a culture of ethical vigilance in the face of rampant funding cuts.
Challenges Facing Medical Research during Funding Shortages
Medical research currently faces unprecedented challenges due to ongoing funding shortages. With the reduction of federal grants and support, research institutions are being forced to prioritize projects, often sidelining those that require rigorous oversight and ethical considerations. This prioritization can lead to a disproportionate focus on commercially viable studies, leaving important yet less profitable areas of research severely underfunded and neglected.
In addition to prioritization issues, funding shortages also limit the capacity of IRBs and research teams to maintain quality oversight. As funding cuts delay the initiation of crucial studies, the ability to conduct thorough reviews becomes compromised, risking participant safety. Researchers, IRB members, and healthcare advocates must unite to address these challenges and advocate for the restoration of essential funding that supports comprehensive oversight and ethical medical research.
Navigating the Consequences of Federal Grant Suspension
The suspension of federal grants, as seen in the current landscape of medical research, leads to a multitude of consequences for both researchers and patients. With many institutions halting studies, the long-term effects on scientific progress and public health are staggering. The ramifications can cause significant disruption not only in research timelines but also in the trust between the scientific community and the public, fostering skepticism toward future medical advancements.
Navigating the aftermath of federal grant suspensions requires both innovation and resilience. Institutions must seek alternative funding sources and collaborate with private entities to bridge the gaps left by federal cuts. However, this raises ethical concerns, as dependence on private funding could compromise the integrity of research priorities. Creative solutions must address these challenges while ensuring that the focus remains on patient safety and ethical research practices.
Frequently Asked Questions
What are the implications of funding cuts in medical research on patient safety?
Funding cuts in medical research significantly undermine patient safety by disrupting the oversight mechanisms, such as institutional review boards (IRBs), that ensure compliance with ethical and safety standards. Reduced funding can lead to inadequate monitoring of clinical trials, heightening risks for participants and undermining public trust in research processes.
How do funding cuts in medical research affect the functionality of IRB patient safety protocols?
Funding cuts can severely limit the resources available for IRBs, which play a crucial role in evaluating and monitoring the safety of clinical research. With restricted funding, these boards may face operational challenges, impacting their ability to provide thorough oversight and support for the rights and welfare of research participants.
What is the impact of funding cuts on the oversight of clinical research?
Funding cuts hinder the capacity for effective clinical research oversight, as the necessary financial resources for robust IRB review and monitoring programs are diminished. This can lead to delays in research, suboptimal patient safety practices, and decreased participation from research sites.
How are healthcare research ethics affected by cuts in medical research funding?
Cuts in medical research funding can lead to compromised healthcare research ethics, as strained resources can impede the ability to adhere to ethical guidelines and protect participant rights. This failure may also result in a lack of training and support for researchers, further risking ethical violations.
What role does NIH funding play in maintaining the integrity of medical research?
NIH funding is essential for supporting rigorous medical research, including safeguarding participant rights and safety through institutional review boards (IRBs). The integrity of medical research heavily relies on sufficient funding for oversight mechanisms, ensuring compliance with ethical regulations.
How do financial limitations affect the collaboration of multiple research sites in medical studies?
Financial limitations from funding cuts can disrupt collaborations among multiple research sites by inhibiting the process of streamlined oversight and governance that systems like SMART IRB provide. This results in delays and complications that can stall innovative medical research projects.
What are the potential long-term effects of reduced medical research funding on public trust in clinical trials?
The long-term effects of reduced medical research funding can include diminished public trust in clinical trials, as funding cuts may lead to a perception of inadequate oversight and safety concerns. This outcome can discourage participation in research, particularly among underrepresented communities.
What steps can be taken to address the challenges posed by funding cuts in medical research?
To address funding cuts in medical research, stakeholders can advocate for increased federal and private funding, establish alternative funding mechanisms, and enhance collaboration between institutions to share resources. Ensuring robust support for IRB operations is also essential for maintaining patient safety and oversight integrity.
| Key Points | Details |
|---|---|
| Funding Cuts Impact | The Trump administration’s funding halt of over $2 billion affects research ensuring patient safety. |
| What is SMART IRB? | A national system for streamlined oversight of medical research across multiple sites, supported by Harvard and other collaborators. |
| Role of IRBs | IRBs review research proposals, protect participant rights, assess risks, and ensure informed consent. |
| Historical Context | IRBs emerged post-World War II to prevent unethical research practices and protect public trust. |
| Impact on Research | Funding cuts halt critical studies, cause delays, and damage trust in the research process. |
| Current Solutions | Despite funding cuts, Harvard Medical School is supporting SMART IRB’s essential work. |
Summary
Funding cuts in medical research pose significant threats to the safety and rights of participants involved in clinical studies. The freeze on federal research grants disrupts established oversight systems, impeding the progress of vital medical research. Without adequate funding, institutions struggle to comply with ethical standards necessary for protecting study participants, leading to delays and increasing public skepticism towards clinical trials. It’s crucial for the health and safety of the American public that we address these funding challenges to maintain the integrity and progress of medical research.