Patient safety in medical research is paramount, as it directly impacts the well-being of participants who volunteer for studies. In recent times, significant challenges such as the halt in medical research funding have raised concerns regarding the efficacy and oversight of clinical trials. The role of Institutional Review Boards (IRBs) is crucial in ensuring that research adheres to strict ethical standards while safeguarding research participant rights. Unfortunately, disruption in funding can hinder necessary clinical research safeguards, leading to potential risks for volunteers involved in these critical studies. As we explore the implications of NIH funding impact and its relationship with patient safety, it becomes increasingly clear that sustainable research financing is essential for protecting individuals in the quest for medical advancements.
Ensuring the safety of participants in scientific studies is increasingly critical as innovative approaches to health research evolve. The commitment to ethical standards and participant protections necessitates an in-depth understanding of research governance, which has been shaped by historical precedent in biomedical ethics. Collaborative efforts among various institutions, overseen by bodies such as the Institutional Review Board, play a significant role in upholding the rights and welfare of those involved in research initiatives. The urgency to maintain adequate funding for research not only enhances the integrity of these studies but also reinforces trust within the community of research participants. By prioritizing patient safety in clinical investigations, the path toward groundbreaking medical discoveries can be navigated responsibly and ethically.
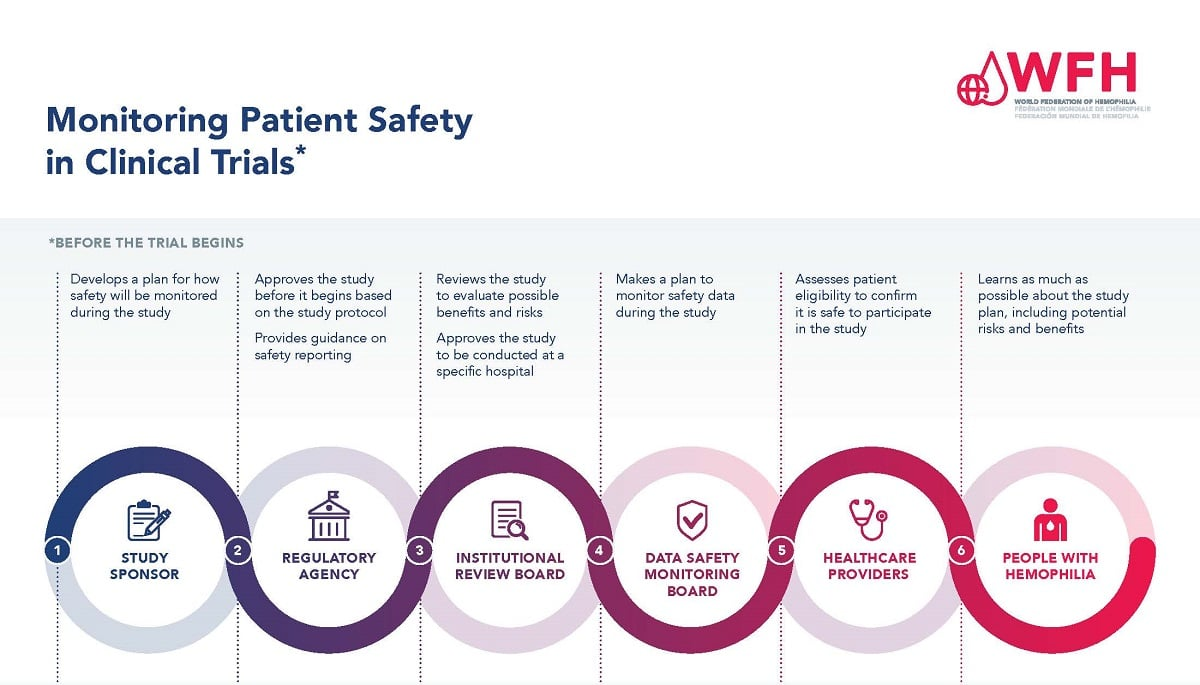
The Importance of Patient Safety in Medical Research
Patient safety is paramount in medical research, and it serves as the cornerstone of ethical clinical trials. Safeguarding participants involves thorough oversight and regulatory measures orchestrated by Institutional Review Boards (IRBs). These boards scrutinize research proposals to ensure they align with ethical standards and prioritize participant welfare. When funding for research is disrupted, as seen with the recent cuts affecting major institutions like Harvard, the mechanisms that protect patients can falter. Inadequate funding can lead to rushed studies and insufficient oversight, exacerbating risks for participants who are already vulnerable.
Moreover, maintaining an environment that upholds patient safety is critical for the trust between researchers and the community. Ethical research practices not only comply with regulatory requirements but also resonate with the values of informed consent and mutual respect. When studies lack proper funding, the pressure to meet deadlines may compromise the thoroughness of these essential processes, ultimately endangering participant safety. Thus, consistent funding is crucial not just for scientific progress but for maintaining the ethical integrity of medical research.
Impact of NIH Funding on Clinical Research Safeguards
NIH funding plays a vital role in supporting the rigorous safeguards established to protect the rights of research participants. Such funding often covers the costs associated with IRB reviews and participant monitoring, ensuring that studies adhere to established ethical standards. The NIH policies that were expanded in recent years, mandating single IRB oversight for multisite studies, illustrate a commitment to streamlining safety protocols while preserving the welfare of participants across different research sites. However, any interruption in this funding threatens to destabilize these safeguards.
Without adequate NIH funding, institutions may struggle to maintain their IRBs, leading to potential inadequacies in the review processes. This jeopardizes not only the integrity of ongoing studies but also the trust participants place in researchers. The loss of funding represents a larger concern about the future of clinical research; it casts doubt on the ability of institutions to uphold the rigorous standards necessary to protect participants. This situation emphasizes the need for consistent investment in medical research to sustain ethical conduct and participant safety.
Challenges Faced by Institutional Review Boards (IRBs) in Research Oversight
Institutional Review Boards (IRBs) serve a critical function in overseeing clinical research, ensuring that participant rights and safety are prioritized at every step. They meticulously examine study proposals, focusing on informed consent, risk assessments, and ethical considerations that arise throughout the research lifecycle. When funding cuts threaten operations, IRBs may struggle to maintain their essential functions, leading to delays in study approvals and oversight. This disruption can have cascading effects, prolonging research timelines and potentially compromising participant safety.
Moreover, the pressure on IRBs can result in a reduction of resources for training and educational programs that are vital for keeping IRB members and researchers informed of the latest ethical standards and regulations. As studies become more complex and collaborative, such knowledge becomes even more crucial. The implications of underfunded IRBs extend beyond logistical setbacks; they signal a breakdown in the ethical framework that governs human research, posing a risk to the very individuals the research aims to benefit.
Historical Context of Ethical Standards in Medical Research
The evolution of ethical standards in medical research has been shaped by historical events that revealed the profound risks associated with unethical practices. Major incidents, such as the Tuskegee Syphilis Study and unethical medical experiments during World War II, highlighted the necessity for robust oversight mechanisms like IRBs. The response to these atrocities was the implementation of strict regulations designed to protect study participants, ensuring that their welfare is prioritized. However, disruptions in research funding can compromise the systems that monitor and uphold these ethical standards.
Today, understanding this history is essential in evaluating the current landscape of medical research. While significant strides have been made in protecting participant rights, the ramifications of funding cuts are stark. When resources dwindle, the vigilance needed to uphold ethical standards may wane, risking a return to less scrupulous practices. Continuous education and funding for oversight mechanisms remain critical to prevent such regression and ensure that history does not repeat itself.
The Role of Research Participant Rights in Safeguarding Health
Research participants possess fundamental rights that must be safeguarded throughout the medical research process. Their right to informed consent, protection from harm, and confidentiality must be universally upheld, ensuring that they are fully aware of the risks and benefits associated with their involvement. Institutional Review Boards (IRBs) enforce these rights by meticulously reviewing research protocols, thereby serving as gatekeepers of ethical standards. Funding cuts can jeopardize these protections, as IRBs may be left without sufficient resources to conduct thorough reviews or monitor ongoing studies.
Protecting participant rights is critical not only for ethical compliance but also for maintaining public trust in medical research. When participants feel secure in their rights, they are more likely to engage in studies, fostering an environment conducive to medical advancements. Conversely, when institutional support falters due to funding disruptions, this critical trust may be eroded, deterring individuals from participating in future studies. Preservation of participant rights is fundamental to the integrity of clinical research, emphasizing the need for consistent support and oversight.
Challenges to Clinical Research Funding and Community Trust
The halt in research funding can lead to an erosion of trust between communities and researchers, significantly impacting future clinical studies. When funding agencies cut grants, promising research initiatives may be abandoned, leaving communities skeptical of researchers’ intentions and capabilities. This skepticism can become a barrier to participation in clinical trials, which are vital for advancing medical science and improving health outcomes. Researchers must recognize the importance of transparency and engagement in rebuilding and maintaining trust within their communities.
Moreover, the repercussions of funding cuts extend beyond immediate research projects—they threaten the foundational relationships between researchers and the communities they serve. Long-term effects can include a diminished willingness from population segments to partake in health studies, directly impacting the validity and inclusiveness of clinical research. Addressing these challenges requires a commitment to ethical engagement, ensuring communities are adequately informed about the research purpose and the safeguards in place to protect their interests.
The Significance of Multi-Site Research Collaborations
Multi-site research collaborations have become a central strategy for advancing medical knowledge, especially for complex studies requiring diverse participant pools. Such collaborations allow for the integration of different expertise and resources, which can enhance research quality and accelerate the pace of discovery. However, when funding is cut, the ability of institutions to collaborate efficiently significantly diminishes. Without the necessary financial backing, studies may face delays, and more institutions may be barred from joining ongoing research efforts, creating obstacles to innovation.
The establishment of systems like SMART IRB has facilitated the collaborative process by allowing a single IRB to oversee multiple sites, decreasing administrative burdens while ensuring participant safety. Yet, funding interruptions can impede these benefits, disrupting the collaborative network that champions patient-centric research. The long-term implications of such disruptions can stifle scientific progress and lessen the overall impact of collaborative research efforts on public health.
Federal Policies and Their Influence on Research Integrity
Federal policies play a crucial role in shaping the ethical landscape of medical research, influencing everything from funding allocations to oversight structures like IRBs. Recent changes to policies, including the mandate for single IRB reviews for multisite studies, have been designed to streamline ethical oversight and enhance participant protection. However, as seen with current funding reductions, these policies are vulnerable to the fluctuations of federal support, potentially compromising their efficacy and the integrity of research.
The relationship between federal policy and research integrity is critical, especially in times of financial uncertainty. The ability of IRBs and researchers to navigate the complexities of ethical compliance hinges on accessible funding and institutional support. As funding becomes scarcer, the risks associated with policy implementation grow, threatening not only research integrity but also the well-being of those who volunteer to participate. Continuous evaluation and commitment to funding are essential to uphold the standards established through federal policies and ensure ongoing progress in medical research.
Long-Term Consequences of Research Funding Cuts on Public Health
The ramifications of cuts to medical research funding extend far beyond immediate disruptions; they pose long-term threats to public health. With fewer resources available to support clinical trials, the pace of medical advancements may slow, delaying the introduction of new treatments and therapies that could benefit patients. As ongoing studies are terminated or postponed, opportunities to address pressing health challenges diminish. This stagnation can further erode public confidence in the research community, making individuals hesitant to partake in future studies.
Moreover, the long-term impact on public health is profound, as research failures can lead to suboptimal health outcomes for populations who rely on advancements in medical science. Funding cuts can reduce the diversity and scope of clinical research, potentially leaving critical health disparities unaddressed. Maintaining a robust funding ecosystem is vital to ensuring that scientific inquiry continues to evolve, public health challenges are met with innovative solutions, and the health of communities is prioritized.
Frequently Asked Questions
How does patient safety in medical research rely on Institutional Review Boards (IRBs)?
Patient safety in medical research is ensured through Institutional Review Boards (IRBs), which meticulously review research proposals to protect participants’ rights and welfare. They assess risks, ensure informed consent is obtained, and monitor adverse events. By enforcing ethical standards, IRBs act as a safeguard for the integrity of medical research.
What is the impact of NIH funding on patient safety in medical research?
NIH funding plays a crucial role in enhancing patient safety in medical research by supporting studies that must undergo rigorous IRB review. This financial support ensures compliance with ethical standards, facilitating safe practices in research involving human participants. With proper funding, research institutions can uphold participant rights and maintain high safety standards.
How do clinical research safeguards protect patients involved in medical studies?
Clinical research safeguards are vital for protecting patients in medical studies. They include comprehensive IRB oversight, monitoring for adverse events, and adherence to informed consent protocols. These measures, coupled with sufficient research funding, ensure a controlled environment where patient safety is the top priority, fostering trust in medical research.
What rights do research participants have to ensure their safety in medical studies?
Research participants have rights that are essential for their safety, including the right to informed consent, the right to withdraw from the study at any time, and the right to be informed about potential risks and benefits. These rights are upheld by the ethical oversight of IRBs and are critical to the integrity of patient safety in medical research.
Why is oversight important for patient safety in multi-site medical research studies?
Oversight is crucial for patient safety in multi-site medical research studies as it ensures a standardized review process that protects participants across all sites. The use of a single Institutional Review Board (sIRB) for these collaborative studies streamlines safety protocols and ensures consistent ethical standards are met, directly enhancing the protection of research participants.
| Key Points | Details |
|---|---|
| Halt in Funding | Over $2 billion in federal research grants to Harvard have been frozen, impacting patient safety efforts. |
| SMART IRB | A national system for the oversight of medical research at multiple sites, aimed at safeguarding participant rights and welfare. |
| Role of IRBs | Institutional Review Boards (IRBs) ensure compliance with laws and protect research participants from harm. |
| Impact of Cuts | Funding cuts threaten ongoing research and pause studies, potentially increasing patient risk and public distrust. |
| Historical Context | Past medical ethics violations highlight the need for strict oversight and patient protection mechanisms. |
Summary
Patient safety in medical research is at significant risk due to the recent halt in funding for vital oversight systems. The freeze on federal research grants disrupts critical efforts aimed at safeguarding patients participating in clinical studies, highlighting the fragility of these protections. The workings of Institutional Review Boards (IRBs) are essential to maintaining ethical standards and ensuring that research meets regulatory requirements. Without adequate funding and support, the safety and welfare of research participants may be compromised, leading to an erosion of public trust in medical research. As we navigate these challenges, the commitment to uphold the highest standards of patient safety must remain paramount.